Electrochemical energy storage has become one of the most important research areas among many different engineering disciplines like materials engineering, chemical engineering and electrical engineering. In addition to this, sometimes, we see the term “supercapacitors” which is a bridge between conventional capacitors and batteries.
In this blog post, it has been aimed to provide general concepts about supercapacitors given by a senior researcher chemical engineering student. It is believed that this technology can be improved with different engineering approaches and form a fundamental building block of modern electrification.
Understanding Supercapacitors and Their Role in Energy Storage
1. The Global Energy Storage Need in the World
The rapid growth of renewable energy sources such as solar and wind power, energy storage has become a very important part of modern energy. As we all know, renewables are intermittent, effective storage technologies are needed to balance supply and demand, stabilize the grid, and ensure a continuous power supply. At this point, we are able to observe the importance of sustainable energy storage solutions because of the global shift toward electrification with technologies like electric vehicles, portable electronics, and smart grids.
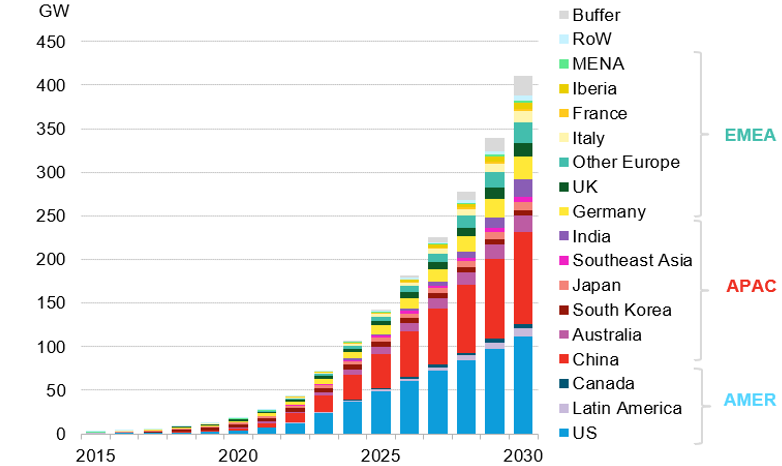
Figure 1. Global Cumulative Energy Storage Installations 2015 – 2023. (BloombergNEF – Global Energy Storage Market Records Biggest Jump Yet https://about.bnef.com/insights/clean-energy/global-energy-storage-market-records-biggest-jump-yet/)
2. Energy Storage Methods
Energy is a fundamental term in our life and its storage methods are a great part of the renewable energy. Energy can be stored in various forms: mechanical (pumped hydro, flywheels, compressed air), thermal (molten salts, phase change materials), chemical (batteries, hydrogen), and electrochemical (supercapacitors, flow batteries). Each of these methods have many different advantages and disadvantages in terms of application areas, depending on factors such as response time, energy density, cost, and scalability.
3. Conventional Capacitors
Conventional capacitors are devices based on the very basic principles of physics and are used to store charge. They store energy through the separation of electric charges across two conductive plates separated by a dielectric material. They can charge and discharge rapidly but store relatively small amounts of energy.
4. Supercapacitors
Supercapacitors having other name of ultracapacitors, bridge the gap between conventional capacitors and batteries. They are able to deliver high power density, charge within seconds, and have long cycle life. However, their energy density is lower than that of batteries, which limits their use in long-term energy storage. In addition to this their energy densities are higher than conventional capacitors which makes them a game changer technology. They are ideal for applications which require rapid charge–discharge cycles.
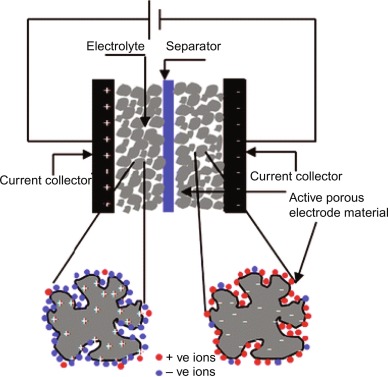
Figure 2. Schematic representation of electric double layer capacitors (EDLCs). Zhou, L., Li, C., Liu, X., Zhu, Y., Wu, Y., & van Ree, T. (2018). Metal oxides in supercapacitors. In S. S. Amghouz & S. J. Patole (Eds.), Metal oxides in energy technologies (pp. 169–203). Elsevier.
5. Classification of Supercapacitors
There are different types of supercapacitors classified into three main types based on their energy storage mechanism: Electric Double Layer Capacitors (EDLCs), Pseudocapacitors and Hybrid Supercapacitors.
6. Electric Double Layer Capacitors (EDLC)
EDLCs store energy by electrostatic charge separation at the electrode–electrolyte interface with the mechanism of adsorption. There are not chemical reactions happening inside EDLC and instead of this, ions accumulate on the surface of porous carbon electrodes. The most common materials used are activated carbon, carbon nanotubes, and graphene. EDLCs offer high power density and excellent cycle stability.
7. Pseudocapacitors
Pseudocapacitors are a class of supercapacitors having faradaic processes which mean they have fast and reversible redox reactions at or near the surface of the electrode materials. Transition metal oxides (like RuO₂, MnO₂) and conducting polymers (such as polyaniline and polypyrrole) are often used. They provide higher energy density than EDLCs but may have shorter lifetimes due to material degradation over cycles.
8. Hybrid Supercapacitors
Hybrid supercapacitors are a combination of EDLCs and pseudocapacitors which make them closer to batteries in terms of mechanism. Typically, one electrode has a battery (faradaic reaction) type behaviour and the other like a capacitor (non-faradaic charge storage). This design aims to achieve a balance between high power density and high energy density, making hybrids attractive for advanced applications like electric vehicles.
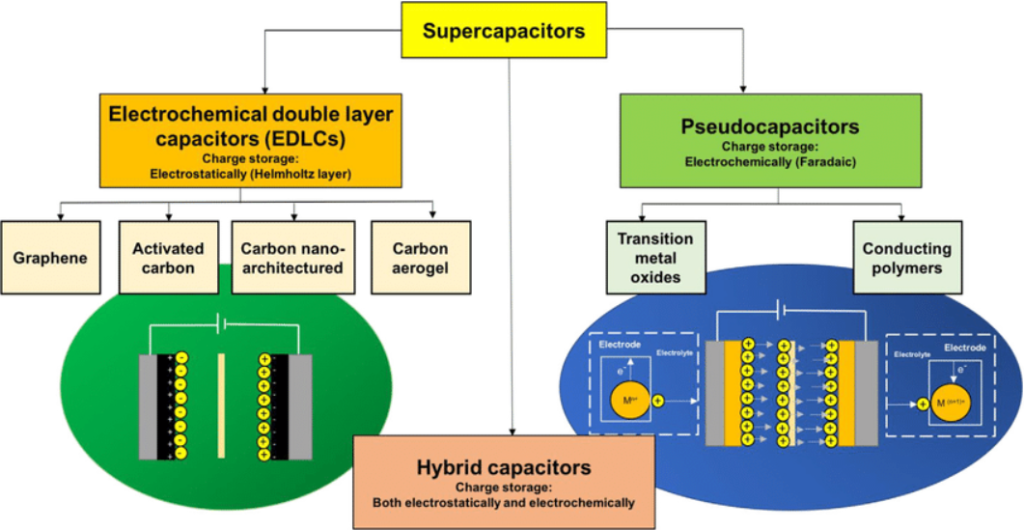
Figure 3. A general classification of supercapacitors. Abdah, M. A. A. M., Azman, N. H. N., Kulandaivalu, S., & Sulaiman, Y. (2019). Review of the use of transition-metal-oxide and conducting polymer-based fibres for high-performance supercapacitors. Materials & Design, 186, 108199.
9. Energy Density and Power Density Comparison (Ragone Plots)
Ragone plots are graphical tools used to compare energy storage devices in terms of their energy and power densities. The main comparison can be made from a ragone plot can be summarized as batteries provide high energy but moderate power, capacitors provide high power but low energy, and supercapacitors occupy the intermediate region—offering a compromise between the two. This situation is generally valid almost for all ragone plots.
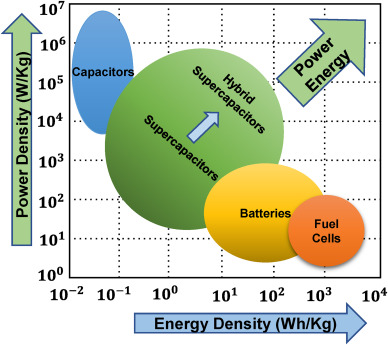
Figure 4. Ragone plot for energy and power density comparison. Ghosh, S., Yadav, S., Devi, A., & Thomas, T. (2022). Techno-economic understanding of Indian energy-storage market: A perspective on green materials-based supercapacitor technologies. Renewable and Sustainable Energy Reviews, 161, 112412.
10. Properties and Performance Metrics of Supercapacitors
Key performance parameters of supercapacitors include specific capacitance, energy density, power density, equivalent series resistance (ESR), and cycle stability. Materials, electrode structure, and electrolyte type strongly influence these metrics. Optimizing these factors is critical for improving performance and expanding the applications.
11. Battery and Supercapacitor Comparison
The main difference between batteries and supercapacitors are the energy storage mechanisms. Batteries store energy through chemical reactions and supercapacitors store it electrostatically or via surface redox reactions. Batteries have higher energy density but slower charging and limited cycle life. Supercapacitors, in contrast, charge very fast and last for hundreds of thousands of cycles but hold less energy. Thus, hybrid systems that combine both are often used to achieve complementary performance.
12. Applications of Supercapacitors
Because of their high power densities, supercapacitors are used in various fields. These applications are generally based on fast charge discharge. regenerative braking systems in electric and hybrid vehicles, backup power for electronics, renewable energy systems, and portable electronic devices. They also serve in power smoothing, load leveling, and peak power assistance due to their high power capability.
13. Conclusions
Supercapacitors are an important class of electrochemical energy storage that complements batteries and other systems. There are a lot of articles about them and the research and development studies are ongoing. Research focuses on developing new electrode materials, optimizing electrolytes, and designing hybrid devices to enhance both energy and power densities. As global energy demand continues to rise, supercapacitors will play an increasingly vital role in sustainable, high-performance energy storage solutions.